10secondi.it
Assessment of progression of COPD: report of a workshopheld in Leuven, 11–12 March 2004M Decramer, R Gosselink, M Rutten-Van Mo¨lken, J Buffels, O Van Schayck, P-A Gevenois,R Pellegrino, E Derom, W De Backer. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
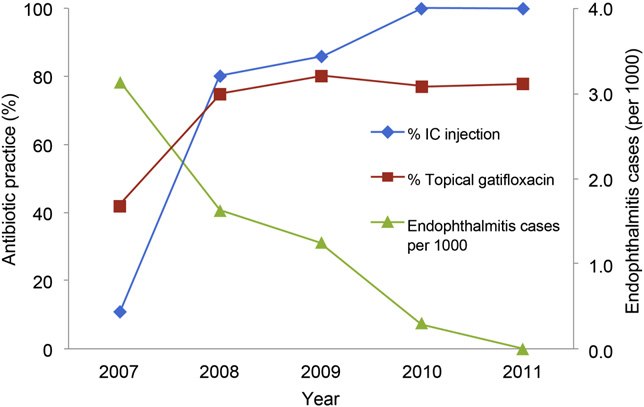 PROPHYLAXIS FOR POSTOPERATIVE ENDOPHTHALMITIS
A suspect endophthalmitis case was considered con-
firmed if clinical endophthalmitis was diagnosed by a KaiserPermanente retinologist based on time of onset, visualacuity, degree of inflammation, vitreous cells, clinical ap-pearance, and the administration of intravitreal antibioticsfor treatment. On querying the retinologists for this study,no cases were believed to represent toxic anterior segmentsyndrome. Cases were considered culture-confirmed if aque-ous or vitreous cultures were positive.
PROPHYLAXIS FOR POSTOPERATIVE ENDOPHTHALMITIS
A suspect endophthalmitis case was considered con-
firmed if clinical endophthalmitis was diagnosed by a KaiserPermanente retinologist based on time of onset, visualacuity, degree of inflammation, vitreous cells, clinical ap-pearance, and the administration of intravitreal antibioticsfor treatment. On querying the retinologists for this study,no cases were believed to represent toxic anterior segmentsyndrome. Cases were considered culture-confirmed if aque-ous or vitreous cultures were positive.