Cfm_bernal
Trade preferences and developing countries: Dealing with inequities Small developing economies and the multilateral Dr.Richard L.Bernal , Small developing economies are often constrained in participating in the negotiation and regulation of multilateral trading rules due to severe cost and resource limitations. This article argues that, despite the costs and difficulties, small st
 Arch Virol (2007)DOI 10.1007/s00705-007-0974-5Printed in the Netherlands
Antiviral activity of arbidol against influenza A virus, respiratory syncytialvirus, rhinovirus, coxsackie virus and adenovirus in vitro and in vivo
L. Shi, H. Xiong, J. He, H. Deng, Q. Li, Q. Zhong, W. Hou, L. Cheng, H. Xiao, and Z. Yang
State Key Laboratory of Virology, Institute of Medical Virology, Wuhan University, Wuhan, P.R. China
Received December 9, 2006; accepted March 18, 2007; published online May 14, 2007# Springer-Verlag 2007
fected with FLU-A (A=PR=8=34 H1N1). Our re-
Arbidol, ethyl-6-bromo-4-[(dimethylamino)-meth-
sults suggest that arbidol has the ability to elicit
yl]-5-hydroxy-1-methyl-2-[(phenylthio)methyl]-in-
protective broad-spectrum antiviral activity against
dole-3-carboxylate hydrochloride monohydrate, is
a number of human pathogenic respiratory viruses.
Arch Virol (2007)DOI 10.1007/s00705-007-0974-5Printed in the Netherlands
Antiviral activity of arbidol against influenza A virus, respiratory syncytialvirus, rhinovirus, coxsackie virus and adenovirus in vitro and in vivo
L. Shi, H. Xiong, J. He, H. Deng, Q. Li, Q. Zhong, W. Hou, L. Cheng, H. Xiao, and Z. Yang
State Key Laboratory of Virology, Institute of Medical Virology, Wuhan University, Wuhan, P.R. China
Received December 9, 2006; accepted March 18, 2007; published online May 14, 2007# Springer-Verlag 2007
fected with FLU-A (A=PR=8=34 H1N1). Our re-
Arbidol, ethyl-6-bromo-4-[(dimethylamino)-meth-
sults suggest that arbidol has the ability to elicit
yl]-5-hydroxy-1-methyl-2-[(phenylthio)methyl]-in-
protective broad-spectrum antiviral activity against
dole-3-carboxylate hydrochloride monohydrate, is
a number of human pathogenic respiratory viruses.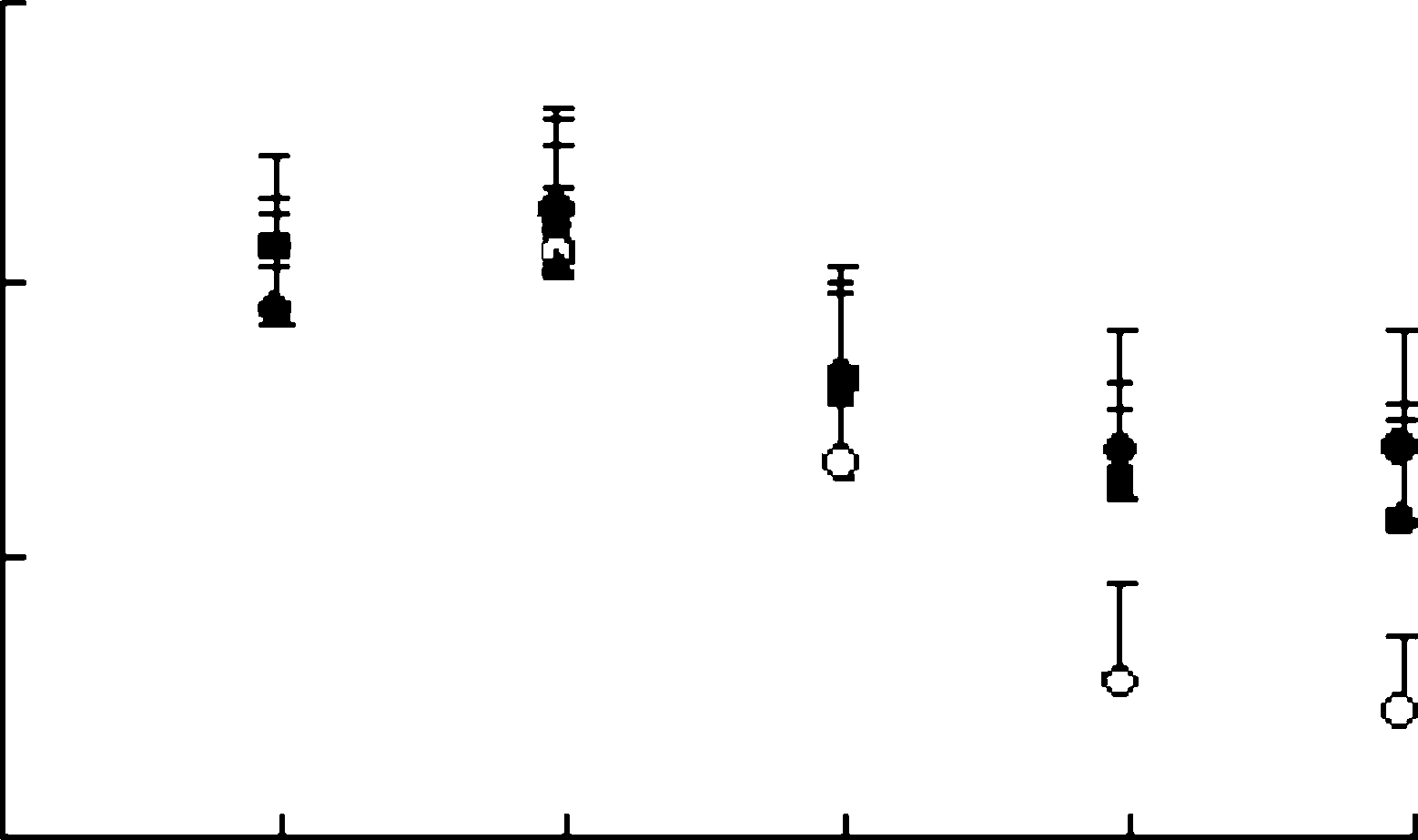 Table 4. Effect of oral treatment with arbidol in mouseinfluenza model
a Mean day to death of mice dying prior to day 21.
Table 4. Effect of oral treatment with arbidol in mouseinfluenza model
a Mean day to death of mice dying prior to day 21.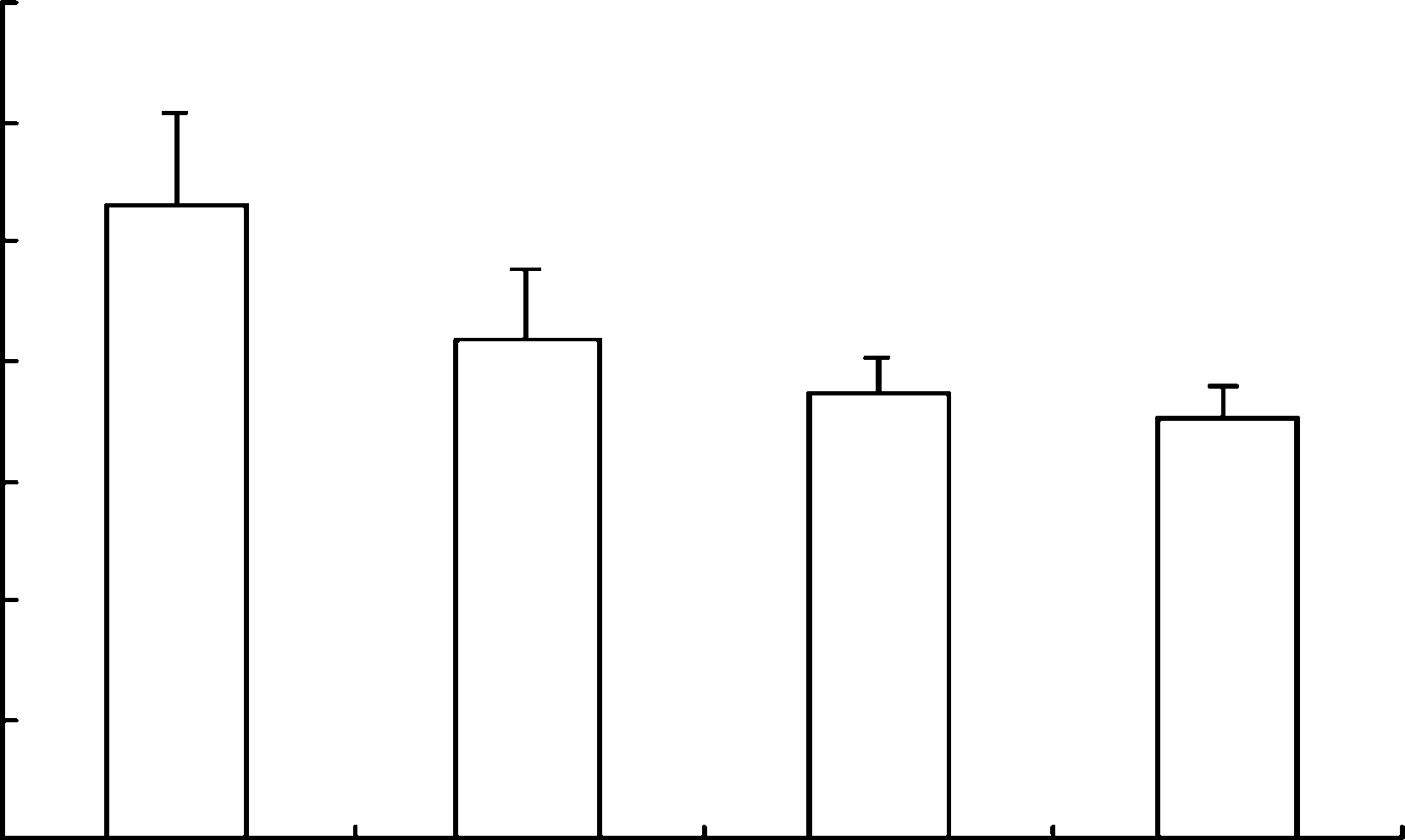 50 or 100 mg=kg=day 24 h before infection withinfluenza virus A=PR=8=34 for 6 days significantlyreduced mean pulmonary virus yields in mice andthe rate of mortality. Our results suggest that arbi-dol, a potent non-specific, broad-spectrum antiviralagent, should deserve our attention in future [3].
50 or 100 mg=kg=day 24 h before infection withinfluenza virus A=PR=8=34 for 6 days significantlyreduced mean pulmonary virus yields in mice andthe rate of mortality. Our results suggest that arbi-dol, a potent non-specific, broad-spectrum antiviralagent, should deserve our attention in future [3].